The U.S. Food and Drug Administration announced a voluntary recall of blood pressure medications due to detected trace amounts of an impurity that could cause cancer.
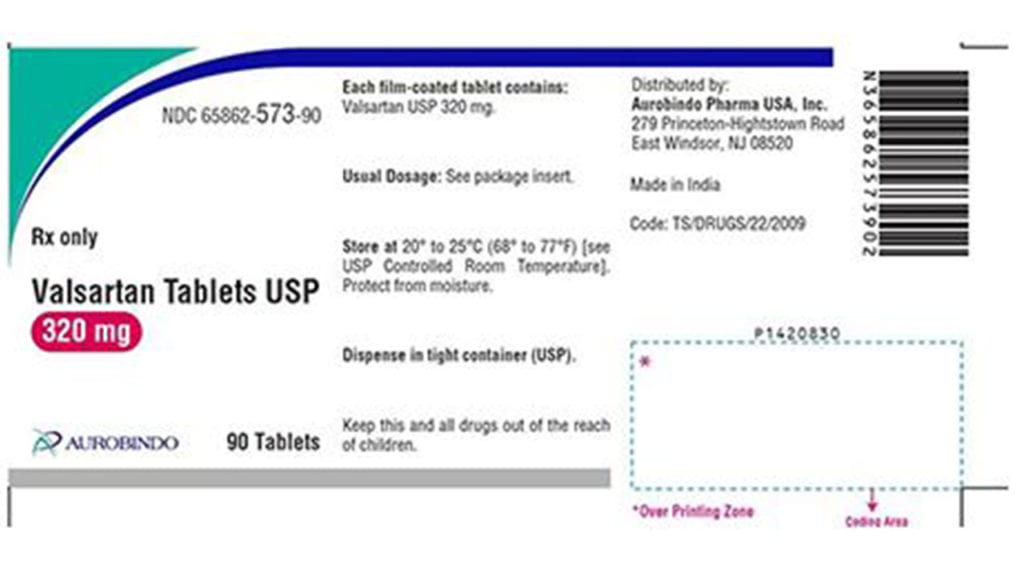
The FDA issued an alert for 80 lots of Amlodipine Valsartan Tablets, Valsartan HCTZ Tablets, USP and Valsartan Tablets. The full list of affected medication can be found here.
According to the FDA, the drugs contained trace amounts of N-nitrosodiethylamine (NDEA), which has been classified as a probable carcinogen by the International Agency for Research on Cancer. Despite that warning, the FDA advises those who take the medication to continue taking it, because patients could be in more danger if they stop taking the medication without an alternative treatment.
If you are taking the recalled medication, talk with your doctor about alternative treatment options before disposing of the medication.
Drugmaker Aurobindo Pharma USA, Inc. has not received any reports of issues from consumers related to these drugs. The product can be identified by looking at the batch or lot number on the bottle.

